Research
The research in the Ramos lab is focused on RNA-binding proteins and their physiopathological roles in vivo. Dr. Ramos has a broad medical and biochemical background, with specific training and expertise in RNA-binding proteins and the creation of genetically-engineered mouse models. From the biochemical perspective, the lab aims to identify novel mRNA targets for RNA-binding proteins and from the medical point of view, we aim to mechanistically understand how RNA binding proteins are involved in pathological condition. Dr Ramos is PI who has co-Investigator and Key Personnel roles on university- and NIH-funded grants. She laid the groundwork for examining the role of ZFP36L2 in vivo and has made several key findings during a NIH K08 award (2012-2017). She has unveiled the role of ZFP36L2 in early embryo development and infertility. Since then, she has expanded to other projects such as to the structural basis of ZFP36L2-mRNA interactions and to other physiological contexts in which ZFP36L2 has a crucial role, such as in T cells and spleen function. These broader directions in understanding how ZFP36L2 functions has developed thanks to UNC being a hub for RNA biochemistry fostering internal and outside collaborations that resulted in several peer-reviewed publications.
Key Scientific discoveries from the Ramos lab:
1. Discovery of the physiological role of ZFP36L2 in mouse female infertility.

Zinc Finger Protein 36-like 2 (ZFP36L2) is an RNA-binding protein that belongs to the small family of zinc finger proteins containing a tandem zinc-binding motif comprising three cysteines followed by one histidine (CCCH). ZFP36, is the prototype of these three family members and has anti-inflammatory properties due to binding to mRNAs containing Adenine-uridine-Rich Elements (AREs) in their 3′-untranslated regions (3’UTR); ARE binding promotes mRNA degradation, ultimately decreasing the levels of cytokines such as tumor necrosis factor (TNF-a) and granulocyte-macrophage colony stimulating factor, GM-CSF. Because at the biochemical level ZFP36L2 can also bind to TNF-a and GM-CSF, it was anticipated that a mouse disrupted for Zfp36l2 expression would exhibit an immunological phenotype. However, using a genetically-engineered mouse (ΔN-Zfp36l2-KD, hypomorphic or knockdown) in which the first exon of Zfp36l2 was deleted, resulting in a truncated protein, ΔN-ZFP36L2, lacking 29 amino acids out of a total of 484, we observed complete female infertility. ZFP36L2 is critical for female fertility because physiological levels are required for normal ovulation rates and early embryonic development, specifically in the progression of the embryo beyond the two-cell stage. These findings represent the first evidence for the involvement of an mRNA-destabilizing protein in the regulation of early embryonic development (Figure 1).
2. Identification and biochemical validation of luteinizing hormone receptor (Lhr) mRNA as the first ovarian ZFP36L2 target.
In 2012, we published in the Journal of Biological Chemistry a seminal, in-depth investigation of the function of ZFP36L2 in fertility. Where we reported that ΔN-ZFP36L2 protein had properties like ZFP36 and WT-ZFP36L2. ΔN-ZFP36L2 shuttles between the cytoplasm and nucleus, it binds to mRNAs containing AREs, and it promotes deadenylation of a model ARE transcript in cell-based co-transfection assays. Thus, suggesting that low expression of ΔN-ZFP36L2 works as a ‘hypomorphic’ or knockdown of WT-ZFP36L2 in vivo. We also identified the first physiological mRNA target for ZFP36L2, i.e., the luteinizing hormone receptor (Lhr) mRNA, and we uncovered new characteristics of this RNA binding protein. ZFP36L2 binds to one of the three ARE-sequences within the 3’ UTR of Lhr mRNA, likely promoting degradation of this Lhr mRNA. Interestingly, other CCCH family members (ZFP36 and ZFP36L1) do not appear to bind this target, suggesting that Lhr transcript is an ZFP36L2-specific mRNA target, potentially explaining the anovulation and oocyte immaturity phenotype we observed in mice.
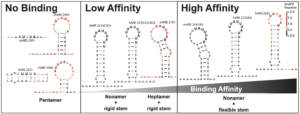
3. Mechanisms affecting ZFP36L2-mRNA interaction, critical role of structural RNA components.
We embarked on an investigation of how RNA secondary structure can affect the regulatory relationship between the RNA-binding protein ZFP36L2 and the binding to the Lhr mRNA that controls LHR levels. Our data determined that this interaction is governed by specific sequence recognition of the ARE motifs in the Lhr 3’ UTR, in both mouse and human transcripts. In addition, a critical component of these regulatory interactions is the structural context of the ARE motif. The ARE sequences and accessibility jointly mediate ZFP36L2 binding ability. We accurately characterized these structures using the chemical RNA structure probing technique known as SHAPE-MaP (Selective 2’ Hydroxyl Acylation with Primer Extension coupled with Mutational Profiling), Figure 2.
4. Sequence and tissue targeting specificity of ZFP36L2 using a novel mouse model (L2-fKO).
The overlap between ZFP36L2-target-mRNAs in different tissues is minimal, suggesting that ZFP36L2-targeting is highly tissue specific. Recently, we developed a novel Zfp36l2 flox global knockout mouse model (L2-fKO), by crossing a Zfp36l2-flox mice where exon 2 of Zfp36l2 is flanked by flox sequences, with the CMV-Cre mouse line from Jackson labs to obtain a novel line (L2-fKO) lacking Zfp36l2 in all tissues. Using RNA-seq from spleen samples from the L2-fKO spleen, we have identified many potential physiological mRNA targets for ZFP36L2 and confirmed that Elavl2 mRNA is a L2-target in the spleen. ELAVL2 is well known RNA-binding protein that seems to play key roles in transcription and mRNA stability. Our discovery of co-regulatory interaction of ZFP36L2 and ELAVL2 on the Elavl2 mRNA (Figure 3) as well as in some other mRNA targets shared between these two proteins, raises an interesting area of further investigation.
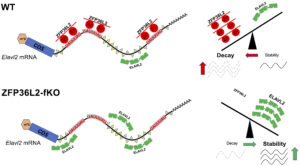
- Role of ZFP36L2 in hematopoiesis using the novel mouse model (L2-fKO).
Also, we are expanding our immunological studies of ZFP36L2 to human T cells in collaboration with other groups, particularly in autoimmune diseases using single-cell resolution and the role of ZFP36L2 in hematopoiesis using our mouse model. This novel area will expand our investigation on other immune cell type precursors. Particularly because the precise mechanism of how ZFP36L2 affects hematopoietic stem cells and progenitor compartments has not been established, we are investigating the mechanism of insufficient hematopoiesis using mice lines deficient in ZFP36L2.
Hematopoietic stem cells (HSC) are responsible for their own self-renewal (maintenance) and for cell differentiation and maturation into three main distinct populations of cells: red blood cells (erythrocytes), white blood cells (immune cells) and platelets (thrombocytes). These maintenance, cell differentiation and maturation processes require precise gene regulation at multiple levels, especially during the cell cycle. A critical and understudied aspect of hematopoietic cell cycle regulation is modulation of mRNA function. Particularly important are the regulatory interactions of RNA-binding proteins with elements within the untranslated regions of messenger RNAs. We began this research theme by identifying an important functional connection between ZFP36L2 and hematopoiesis. Our lab has created a novel mouse model, L2-fKO, in which ZFP36L2 gene has been ubiquitously knockout under the control of a Cre system. These homozygous pups lacking both Zfp36l2 copies die within 2 weeks after birth. They develop, at an early age, severe pancytopenia (extremely low numbers of erythrocytes, immune cells, and thrombocytes) in the peripheral blood, with decreased number of cells in the bone marrow, suggesting a maturation and/or maintenance defect in HSCs (Figure 4). Recently, a direct connection between Diamond-Blackfan anemia and imbalance of ZFP36L2 has been made, thus we suspect ZFP36L2 is involved in the physiopathology of other myelodysplastic syndromes (MDS), particularly in rare subtypes present in children. Our findings may provide new knowledge and opportunities to improve clinical solutions for MDS.
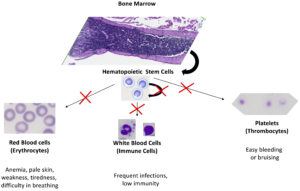
Myelodysplastic syndromes (MDS) encompass a group of clonal hematopoietic stem cell characterized by ineffective hematopoiesis with significant morbidity and high mortality. Prevalence of MDS is currently lacking globally, providing a challenge for healthcare planning and care. In the US, the prevalence of MDS is estimated to be between 60,000 – 170,000 patients. Yearly, about 20,000 new cases are reported in US, comprising one of the most common blood cancers. The incidence tends to increase with age, particularly after the 6th decade of life. MDS is a rare disease in kids, occurring in four out of 1 million children. However, the subtypes of MDS identified in children are somewhat different from the ones common in adults and older people, thus our novel mouse model might shed some light into these rare cases, with poor prognosis in children.
A full list of the lab’s publications can be found here.
LAB DIVERSITY STATEMENT
The Triple E core values of our lab are: Embrace, Educate and Empower
The Ramos Lab is committed to embrace individuals from all backgrounds and groups, including, but not restricted to those from under-represented minorities. We consider the education process as being dynamic and transformative. In ways that that the unforeseen novel knowledge leads a to an empowered society.